- https://www.cancer.net/cancer-types/colorectal-cancer/risk-factors-and-prevention#:~:text=About%2011%25%20of%20all%20colorectal,cancer%20in%20the%20United%20States
- Adler A, Geiger S, Keil A, Bias H, Schatz P, deVos T, et al. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014 Oct 17;14:183
- Australia colorectal cancer statistics. Available at: https://ncci.canceraustralia.gov.au/diagnosis/distribution-cancer-stage/distribution-cancer-stage
- UK colorectal cancer statistics. Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/survival#heading-Three
- US colorectal cancer statistics. Available at : https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html
- https://www.canceraustralia.gov.au/cancer-types/bowel-cancer/awareness
- National Cancer Control Indicators – https://ncci.canceraustralia.gov.au/screening/colorectal-screening-rates/colorectal-screening-rate-participation
- Liles EG, Coronado GD, Perrin N, Harte AH, Nungesser R, Quigley N, Potter NT, Weiss G, Koenig T, deVos T. screening blood test is higher than of a fecal test offered in clinic: A randomized trial. Cancer Treatment and Research Communications. 2017 10: 27-31

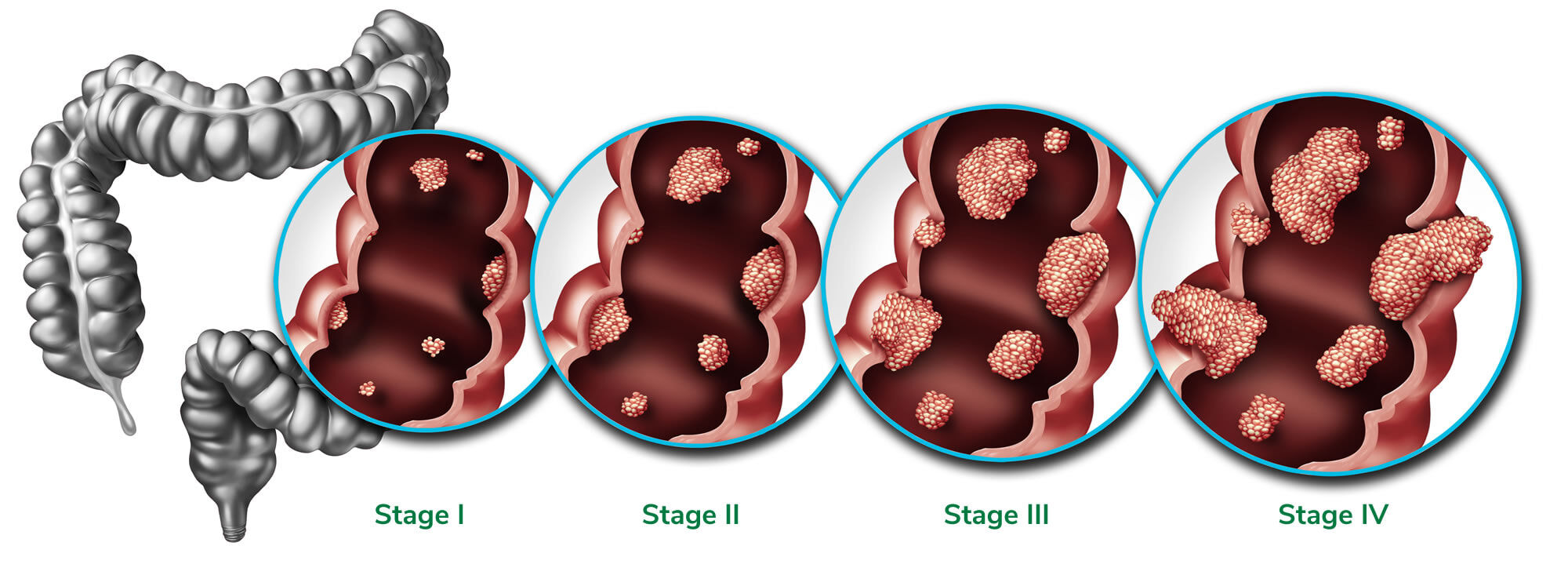
It is well established that the 5-year survival rate for CRC, is greater than 90% when detected early2
ColoSTAT® Symptomatic Clinical Performance
90.7%
89.4%
| ColoSTAT® Sensitivity (%) at each Cancer Stage | ||||
|---|---|---|---|---|
| I 85.0% |
II 95.7% |
III 84.4% |
IV 100.0% |
AA 58.0% |
Internal data, Rhythm Biosciences 2025
Risk factors associated with CRC
A risk factor is any factor that may increase a person’s possibility of developing cancer. Some risk factors are referred to as modifiable, such as lifestyle or environmental risk factors. Others are called non-modifiable, such as inherited factors or whether someone in the family has had cancer.6
The cause of colorectal cancer is not known. Evaluating your risk factors and discussing them with the doctor may help you make more informed lifestyle and health care choices.3
Alternatively, tests like the geneType™ CRC risk assessment assay can identify risk of disease in the future.
Modifiable Risk Factors6

A diet low in fibre

High red meat consumption, especially processed meats

Physical inactivity and obesity

Consumption of alcohol and tobacco

Low blood level of vitamin D
Non-Modifiable Risk Factors6

Personal history of certain types of cancers (ovarian/uterine)

IBD, such as Crohn’s and Ulcerative Colitis

Family history of CRC

Type 2 Diabetes
Aged ≥ 50
A previous diagnosis of polyps/CRC
Participation in CRC screening remains suboptimal despite national programs being in place in many countries worldwide. In Australia, over 50% of eligible individuals remain unscreened for CRC, and this has not changed since the national program was introduced in 2006.7
Observational studies have shown that 78-93% of people offered CRC screening, preferred blood-based tests over faecal tests, with ease and convenience being the main reason for their preference.5
Reasons for preferring blood-based options6:
- Convenience of a blood draw
- Ease / comfort of a blood test
- Lower time requirement vs faecal test
- No special preparation required
Rhythm Biosciences have developed ColoSTAT®, a simple, affordable blood-based test for the detection of CRC in symptomatic patients.
SIMPLE – A blood test like other standard tests the doctor requests. It is designed for convenience and is more hygienic compared to handling traditional stool samples.
ACCURATE – ColoSTAT® is 01% sensitive, which is comparable to current faecal-based tests (FIT). A negative result means that there is a less than 1% chance of colon cancer, helping to avoid unnecessary colonoscopies.
PATIENT FRIENDLY –More convenient than faecal-based tests (FIT).
ColoSTAT® has the potential to:
- Provide an alternative for those unwilling or unable to perform FIT
- It allows doctors to triage patient smarter, prioritising patients most likely to have CRC and reducing colonoscopy wait lists.
- Improve early diagnosis of CRC in symptomatic patients
- Improve survival rates
Intended Use
ColoSTAT® is a quantitative multiplex immunoassay that detects protein biomarkers in human serum. It is intended for people with symptoms of colorectal cancer.
immunoassay test does not diagnose colorectal cancer but is a tool for determining when colonoscopy or another type of diagnostic follow-up is recommended.
A positive result may indicate the presence of colorectal neoplasia and should be followed up with a colonoscopy.
ColoSTAT® is not a replacement for diagnostic colonoscopy, or for surveillance colonoscopy in high-risk individuals.
ColoSTAT® clinical performance
Rhythm’s validation studies demonstrated high sensitivity: ColoSTAT® has a sensitivity of 91% for early stage colon cancer; 58% for advanced adenomas and 99.4% Negative predictive value (NPV)1
References
Contact
Rhythm Biosciences
Bio21 Institute
30 Flemington Road
Parkville VIC 3010
Australia
